The elements of the periodic table sorted by density
click on any elements name for further chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry
Liquid water has a density of about 1 kg/dm 3, making any of these SI units numerically convenient to use as most solids and liquids have densities between 0.1 and 20 kg/dm 3. Kilogram per cubic decimetre (kg/dm 3) gram per cubic centimetre (g/cm 3) 1 g/cm 3 = 1000 kg/m 3; megagram (metric ton) per cubic metre (Mg/m 3). The more standard deviations below 0, indicated as negative numbers, the lower your BMD and the higher your risk of fracture. As shown in the table below, a T-score between +1 and −1 is considered normal or healthy. A T-score between −1 and −2.5 indicates that you have low bone mass, although not low enough to be diagnosed with osteoporosis.
Optical Density Number (Sometimes prefaced with an 'ND' before the number). Your 0.6 GND filter gives you 2-stops of reduction. A 0.3 gives you 1 more stop, a 0.9 gives 3 more, and a 1.2 gives 4 more. I would suggest the 1.2 for a 6-stop reduction when combined, or a 2-stop or 4-stop when used separately. Describe how the concept of density relates to an object's mass and volume. Explain how objects of similar mass can have differing volume, and how objects of similar volume can have differing mass. Explain why changing an object's mass or volume does not affect its density (ie, understand density as an intensive property). Ρ = Density of substance in kgm-3; ρ 0 = Density of pure water (1000 kgm-3 @4°C) SG = Specific gravity (e.g. Pure water = 1) Density of Substance (ρ) This is the measure of the amount of mass per unit volume of a substance. The density is derived by dividing the mass of a quantity of substance by its volume.
| The chemical elements of the periodic chart sorted by: | Density | Name chemical element | Symbol | Atomic Number |
| - Name alphabetically | 0.09 | Hydrogen | H | 1 |
| - Atomic number | 0.18 | Helium | He | 2 |
| - Symbol | 0.53 | Lithium | Li | 3 |
| - Atomic Mass | 0.86 | Argon | Ar | 18 |
| - Electronegativity | 0.9 | Neon | Ne | 10 |
| - Density | 0.97 | Sodium | Na | 11 |
| - Melting point | 1.25 | Nitrogen | N | 7 |
| - Boiling point | 1.43 | Oxygen | O | 8 |
| - Vanderwaals radius | 1.55 | Calcium | Ca | 20 |
| - Year of discovery | 1.63 | Rubidium | Rb | 37 |
| - Inventor surname | 1.7 | Fluorine | F | 9 |
| - Elements in earthcrust | 1.74 | Magnesium | Mg | 12 |
| - Elements in human body | 1.78 | Potassium | K | 19 |
| - Covalenz radius | 1.82 | Phosphorus | P | 15 |
| - Ionization energy | 1.85 | Beryllium | Be | 4 |
Omnigraffle pro 7 10 2018. For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element with the lowest density is Hydrogen and the highest density is Osmium. The unit of density is gr/cm3 (grams per cubic centimeter) for solids and gr/l (grams per liter) or kg/m3 (kilograms per cubic) for gases Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 1.87 | Cesium | Cs | 55 |
| 2.07 | Sulfur | S | 16 | |
| 2.26 | Carbon | C | 6 | |
| 2.33 | Silicon | Si | 14 | |
| 2.34 | Boron | B | 5 | |
| 2.54 | Strontium | Sr | 38 | |
| 2.7 | Aluminum | Al | 13 | |
| 3.00 | Scandium | Sc | 21 | |
| 3.12 | Bromine | Br | 35 | |
| 3.21 | Chlorine | Cl | 17 | |
| 3.59 | Barium | Ba | 56 | |
| 3.75 | Krypton | Kr | 36 | |
| 4.47 | Yttrium | Y | 39 | |
| 4.54 | Titanium | Ti | 22 | |
| 4.79 | Selenium | Se | 34 | |
| 4.93 | Tellurium | Te | 52 | |
| 5.24 | Europium | Eu | 63 | |
| 5.32 | Germanium | Ge | 32 | |
| 5.5 | Radium | Ra | 88 | |
| 5.72 | Arsenic | As | 33 | |
| 5.9 | Xenon | Xe | 54 | |
| 5.91 | Gallium | Ga | 31 | |
| 6.11 | Vanadium | V | 23 | |
| 6.15 | Lanthanum | La | 57 | |
| 6.24 | Iodine | I | 53 | |
| 6.51 | Zirconium | Zr | 40 | |
| 6.68 | Antimony | Sb | 51 | |
| 6.77 | Cerium | Ce | 58 | |
| 6.77 | Praseodymium | Pr | 59 | |
| 6.9 | Ytterbium | Yb | 70 | |
| 7.01 | Neodymium | Nd | 60 | |
| 7.13 | Zinc | Zn | 30 | |
| 7.19 | Chromium | Cr | 24 | |
| 7.3 | Promethium | Pm | 61 | |
| 7.31 | Indium | In | 49 | |
| 7.31 | Tin | Sn | 50 | |
| 7.43 | Manganese | Mn | 25 | |
| 7.52 | Samarium | Sm | 62 | |
| 7.87 | Iron | Fe | 26 | |
| 7.9 | Gadolinium | Gd | 64 | |
| 8.23 | Terbium | Tb | 65 | |
| 8.55 | Dysprosium | Dy | 66 | |
| 8.57 | Niobium | Nb | 41 | |
| 8.65 | Cadmium | Cd | 48 | |
| 8.8 | Holmium | Ho | 67 | |
| 8.9 | Nickel | Ni | 28 | |
| 8.9 | Cobalt | Co | 27 | |
| 8.96 | Copper | Cu | 29 | |
| 9.07 | Erbium | Er | 68 | |
| 9.3 | Polonium | Po | 84 | |
| 9.32 | Thulium | Tm | 69 | |
| 9.73 | Radon | Rn | 86 | |
| 9.75 | Bismuth | Bi | 83 | |
| 9.84 | Lutetium | Lu | 71 | |
| 10.07 | Actinium | Ac | 89 | |
| 10.22 | Molybdenum | Mo | 42 | |
| 10.5 | Silver | Ag | 47 | |
| 11.35 | Lead | Pb | 82 | |
| 11.5 | Technetium | Tc | 43 | |
| 11.72 | Protactinium | Pa | 91 | |
| 11.85 | Thallium | Tl | 81 | |
| 12.02 | Palladium | Pd | 46 | |
| 12.37 | Ruthenium | Ru | 44 | |
| 12.41 | Rhodium | Rh | 45 | |
| 13.31 | Hafnium | Hf | 72 | |
| 13.5 | Curium | Cm | 96 | |
| 13.55 | Mercury | Hg | 80 | |
| 13.67 | Plutonium | Pu | 94 | |
| 14.78 | Berkelium | Bk | 97 | |
| 15.1 | Californium | Cf | 98 | |
| 15.4 | Thorium | Th | 90 | |
| 16.65 | Tantalum | Ta | 73 | |
| 18.95 | Neptunium | Np | 93 | |
| 19.32 | Gold | Au | 79 | |
| 19.35 | Tungsten | W | 74 | |
| 19.84 | Americium | Am | 95 | |
| 20.2 | Uranium | U | 92 | |
| 21.04 | Rhenium | Re | 75 | |
| 21.45 | Platinum | Pt | 78 | |
| 22.4 | Iridium | Ir | 77 | |
| 22.6 | Osmium | Os | 76 | |
| Astatine | At | 85 | ||
| Francium | Fr | 87 | ||
| Einsteinium | Es | 99 | ||
| Fermium | Fm | 100 | ||
| Mendelevium | Md | 101 | ||
| Nobelium | No | 102 | ||
| Rutherfordium | Rf | 104 | ||
| Lawrencium | Lr | 103 | ||
| Dubnium | Db | 105 | ||
| Bohrium | Bh | 107 | ||
| Seaborgium | Sg | 106 | ||
| Meitnerium | Mt | 109 | ||
| Hassium | Hs | 108 | ||
| Darmstadtium | Ds | 110 | ||
| Roentgenium | Rg | 111 | ||
| Ununbium | Uub | 112 | ||
| Ununtrium | Uut | 113 | ||
| Ununquadium | Uuq | 114 | ||
| Ununpentium | Uup | 115 | ||
| Ununhexium | Uuh | 116 | ||
| Ununseptium | Uus | 117 | ||
| Ununoctium | Uuo | 118 |
Click here: for a schematic overview of the periodic table of elements in chart form
Lenntech (European Head Office) Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Density 105
Lenntech USA LLC (Americas) 5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Density 1 0 2 0
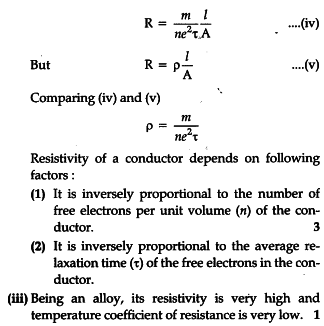
Density 1018 Steel

Density 1018 Steel
Lenntech DMCC (Middle East)
Density 1018 Mild Steel In Si
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Density 1000
Copyright © 1998-2020 Lenntech B.V. All rights reserved
